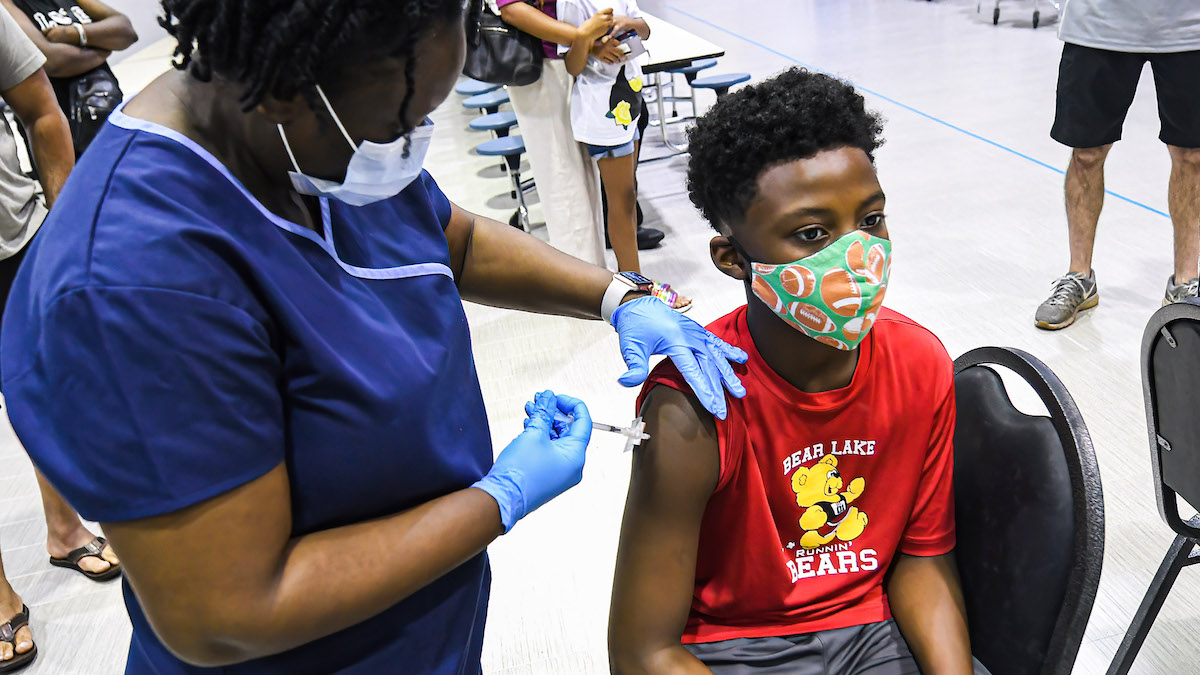
New York City Mayor Bill de Blasio says COVID-19 vaccines could begin being administered to kids age 5-11 early next month if federal health officials grant emergency use authorization of the Pfizer shots for that subset in the coming days.
"We may be starting this on a weekend," de Blasio said Tuesday, indicating that Saturday, Nov. 6 or Sunday, Nov. 7, might be when vaccines could start to be administered for children in that age group.
A key step in the authorization process happened on Tuesday. The FDA's Vaccines and Related Biological Products Advisory Committee reviewed Pfizer's data and heard from regulators in an all-day meeting on the vaccine's safety and efficacy before voting on whether its benefits outweigh any serious potential side effects in children. After the lengthy review, the panel voted to endorse Pfizer's COVID vaccine for 5- to 11-year-olds.
If the FDA goes on to authorize the shots, the Centers for Disease Control and Prevention will make additional recommendations on who should receive them the first week of November. Children could begin vaccinations early next month -- with the first youngsters in line fully protected by Christmas.
Get Tri-state area news and weather forecasts to your inbox. Sign up for NBC New York newsletters.
More Coverage
Full-strength Pfizer shots already are recommended for anyone 12 or older, but pediatricians and many parents are anxiously awaiting protection for younger children to stem infections from the delta variant and help keep kids in school.
A week ago, New York Gov. Kathy Hochul urged parents to start trying to schedule vaccination appointments for their younger children "now," adding, "You don't want to hear the first appointment is in February" when the FDA gives the go-ahead.
It's not clear how many pediatric offices would have accepted vaccine appointments for children not yet federally authorized to receive the shots.
To that point, Dr. Joseph Sellers, a pediatrician and president of the Medical Society of the State of New York, which represents about 30,000 doctors statewide, said it might be difficult or impossible until the logistics are ironed out.
"We don’t know how quickly the vaccine will be in our hands — it’s a different dose. A different colored bottle with a different top so we don’t mess up," Sellers said. "The governor may be making more work for us, but I’m glad that she is."
With 1.5 million kids in that age range statewide, there’s plenty at stake.
Hochul said that she is open to reopening mass vaccination sites, such as the Javits Center in Manhattan, or putting more focus on school vaccination hubs.
Asked last week whether she supports mandating the COVID vaccine for eligible children, Hochul said that she's "looking at it a little more short term," and that would be something to be debated for Fall 2022.
For his part, de Blasio has said he does not support mandating COVID vaccines for eligible kids in New York City public schools at this time.
Last week, the FDA review affirmed results from Pfizer showing the two-dose shot was nearly 91% effective at preventing symptomatic infection in young children. Researchers calculated the figure based on 16 COVID-19 cases in youngsters given dummy shots versus three cases among vaccinated children.
There were no severe illnesses reported among any of the youngsters, but the vaccinated ones had much milder symptoms than their unvaccinated counterparts.
Most of the study data was collected in the U.S. during August and September, when the delta variant had become the dominant COVID-19 strain.
The FDA review found no new or unexpected side effects. Those that did occur mostly consisted of sore arms, fever or achiness.
However, FDA scientists noted that the study wasn't large enough to detect extremely rare side effects, including myocarditis, a type of heart inflammation that occasionally occurs after the second dose.
Not sure how the process works? Check out our handy tri-state vaccine site finder and FAQs here
New York City and New Jersey Vaccine Providers
Click on each provider to find more information on scheduling appointments for the COVID-19 Vaccine.
Data: City of New York, State of New Jersey • Nina Lin / NBC