
As the U.S. races to deliver more life saving vaccines after Moderna's two-dose shot was approved for emergency use, Congress is up against the clock to deliver economic lifelines for Americans who are struggling from the pandemic's economic fallout.
For months, negotiations in Congress have failed to produce a new Covid stimulus deal. But on Saturday night, lawmakers finally reached a compromise on a major hurdle — the Federal Reserve's emergency lending powers — paving the way for votes as soon as Sunday.
Lawmakers had to pass last-minute emergency funding on Friday to avert a government shutdown and create time for another round negotiations over the weekend.
If lawmakers do not reach a deal by 12:01 a.m. ET Monday morning, the government will shut down.
As Congress fumbles the economic response to the pandemic, there are also growing questions about Operation Warp Speed's logistical plan for delivering vaccine doses. Several U.S. states are reporting that their allotment of doses has either been reduced or delayed.
U.S. Army Gen. Gustave Perna, who oversees logistics for Operation Warp Speed, said the confusion was due to a "planning error" in which the initial vaccine numbers he provided to the states were higher than the amount of doses actually available for release.
As the government struggles with its response, the suffering from the pandemic continues to mount. More than 2,800 deaths attributed to the virus were recorded Friday in the U.S., while the nation reported more than 249,000 new infections
The following data is from Johns Hopkins University:
- U.S. deaths: more than 313,000
- U.S. infections: more than 17.4 million
- Global deaths: 1.67 million
- Global infections: 75.7 million
Here's what you need to know:
- Congress reaches major compromise on Fed lending for stimulus package
- CDC advisory panel backs Moderna vaccine after FDA authorization
- Army general apologizes for cuts to vaccine allotments for U.S. states
- Moderna vaccine doses start arriving Monday along with Pfizer shipments
- Senate might not vote on stimulus for days as clock ticks
- Here's where the stimulus talks stand
- U.K. identifies new coronavirus strain that spreads quicker
- London goes into stricter lockdown ahead of Christmas
Lawmakers reach Fed lending compromise for Covid-19 stimulus package
Money Report
Senior lawmakers reached a compromise over the Federal Reserve's emergency lending powers late Saturday night, overcoming a major hurdle that prevented Congress from completing a $900 billion coronavirus relief package earlier in the week, according to multiple sources.
A last-minute roadblock emerged on Friday as Democrats accused Republicans, namely Pennsylvania's Sen. Pat Toomey, of attempting to encumber the incoming Biden administration by cutting off the Federal Reserve's emergency lending abilities created by the CARES Act meant to protect the already battered economy.
"...Democrats have agreed to a version of Sen. Toomey's important language," a spokesman for Senate Majority Leader Mitch McConnell told NBC News.
Compromise language is being finalized and any remaining items are expected to be worked out overnight, according to aides.
—NBC News
Senate adjourns without a stimulus deal, but will reconvene tomorrow
The Senate has adjourned for the evening without a Covid stimulus deal, but will reconvene tomorrow around 1 p.m. ET as Congress races against a self-imposed deadline.
The House of Representatives, meanwhile, will not vote on a stimulus package until 1 p.m. ET at the earliest on Sunday. Congress has tied stimulus to broader government funding legislation and needs to reach a deal by 12:01 a.m. ET Monday to avoid a government shutdown.
Lawmakers expressed optimism Saturday morning, but talks have gotten bogged down over a proposal from Sen. Pat Toomey, R-Pa., to end the Federal Reserve's emergency lending powers that allow it to provide loans to small and medium-sized business as well as state and local governments.
Senate Minority Leader Chuck Schumer, D-N.Y., and Toomey have met at least twice Saturday evening to try and find a path forward.
—Spencer Kimball
CDC: Don't take vaccine if you've had severe allergic reaction to any ingredient
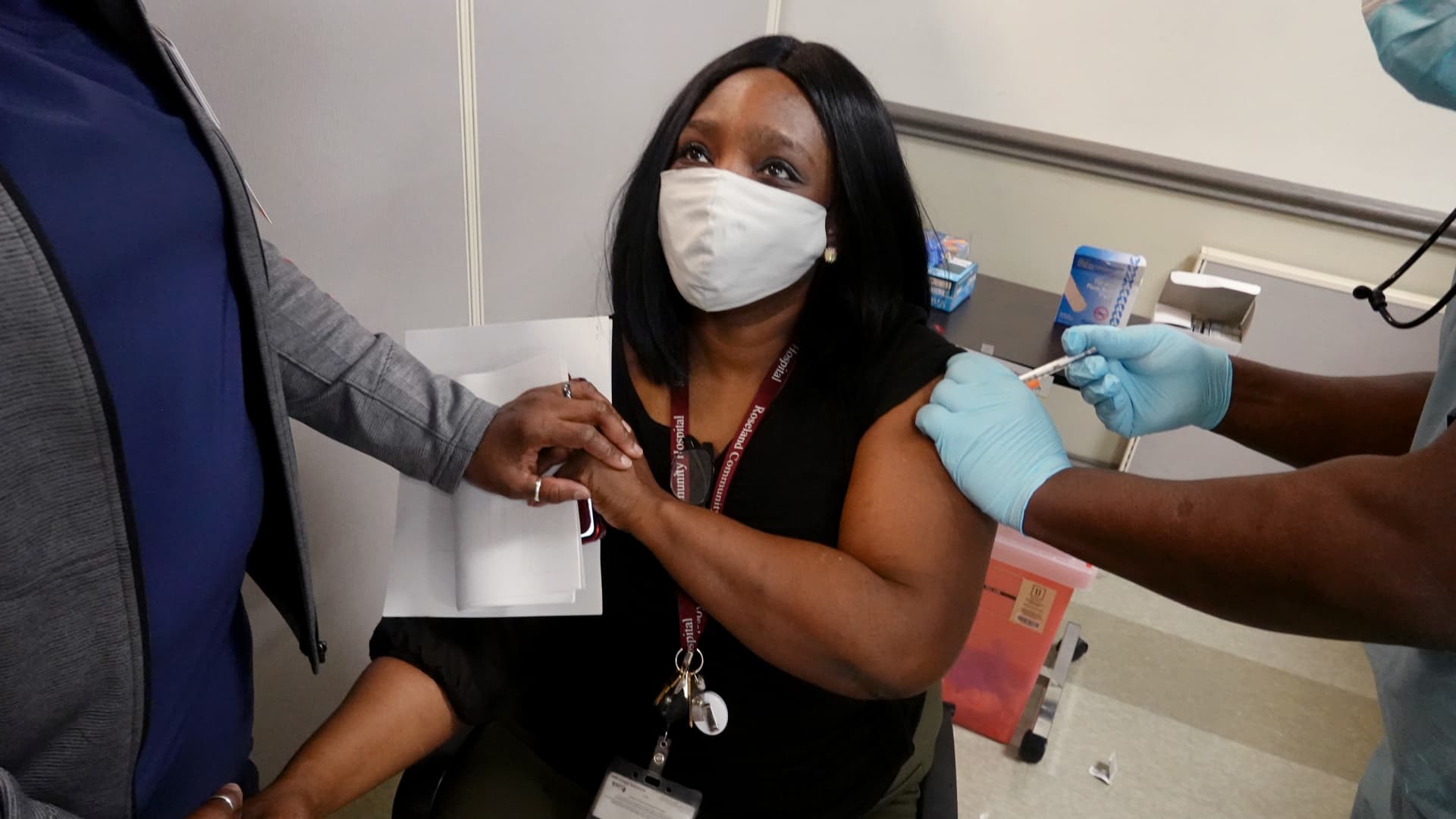
The U.S. Centers for Disease Control and Prevention is recommending that people not get a Covid-19 vaccine if they have had a severe allergic reaction to any individual ingredient in that vaccine.
People who have severe reactions to other vaccines should consult with their doctors to figure out if they should get a vaccine for the virus, the CDC said. People who have a severe allergic reaction after getting an initial dose of a Covid vaccine should not get a second shot of it, the agency said.
The recommendation comes after the CDC has received reports of some people having severe allergic reactions to coronavirus vaccines. The agency considers a reaction severe if it requires the use of epinephrine, an EpiPen or a hospital visit.
Vaccination providers are expected to report cases of severe allergic reactions to Covid shots in the CDC and the Food and Drug Administration's Vaccine Adverse Event Reporting System.
"Reports of adverse events that are unexpected, appear to happen more often than expected, or have unusual patterns are followed up with specific studies," the CDC said.
—Jordan Novet
Pfizer says vaccines must receive 'certificate of analysis' two days prior to distribution, after Army general apologizes for shortfalls
Several U.S. states have reported that the number of Pfizer vaccine doses they were promised by the federal government has been significantly reduced, and although the logistics chief for Operation Warp Speed has taken responsibility for the confusion, it's still not entirely clear what caused the shortfalls.
Pfizer, however, explained in a statement to CNBC that each lot of its vaccines must receive "certificates of analysis" at least 48 hours prior to distribution under the company's emergency use authorization with the U.S. Food and Drug Administration.
Pfizer's statement comes after U.S. Army Gen. Gustave Perna, the OWS logistics chief, apologized to the states for what he called a "planning error."
"The mistake I made is not understanding with exactness — again my responsibility — on all the steps that have to occur to make sure the vaccine is releasable," Perna said at a press briefing.
It wasn't made clear whether the certificates were the reason for the discrepancy between the numbers Perna originally gave the states and what was actually available for release.
A spokesperson for OWS was not immediately available for comment.
"Our sites continue to produce more vaccine and at an increasing rate as we gain more experience with the mRNA vaccine manufacturing process," Pfizer spokeswoman Kim Bencker told CNBC in an emailed statement following Perna's apology.
Pfizer has "millions" of doses in its warehouses ready to ship once it receives word from Operation Warp Speed, and the company continues to coordinate closely with Perna regarding future shipments, Bencker said.
"We remain confident in our ability to deliver up to 50 million doses globally this year and up to 1.3 billion next year," Bencker said.
— Noah Higgins-Dunn
Bernanke says Fed lending authorities should remain intact in stimulus package

Former Federal Reserve Chairman Ben Bernanke is calling for the central bank's emergency lending authorities to remain in place to fight future crises.
Bernanke's statement comes as congressional lawmakers battle over a Republican-backed provision in the Covid-19 stimulus package that would end the Fed's emergency lending powers.
Read the full statement:
I strongly support the passage of new aid for families and businesses suffering from the effects of the pandemic. However, it is also vital that the Federal Reserve's ability to respond promptly to damaging disruptions in credit markets not be circumscribed.
The relief act should ensure, at least, that the Federal Reserve's emergency lending authorities, as they stood before the passage of the CARES Act, remain fully intact and available to respond to future crises.
— Emma Newburger
Battle over Fed’s lending powers is holding up stimulus deal
Disagreement by congressional leaders over a provision on the Federal Reserve's lending powers is hampering progress on a Covid-19 stimulus deal.
Senate Majority Leader Mitch McConnell, R-Ky., told GOP Senators on a conference call on Saturday that he strongly supports Pennsylvania Sen. Pat Toomey's provision that would end the Fed's emergency lending powers, a source on the call told NBC News. Those powers allow it to lend to small and medium-sized businesses as well state and local governments.
McConnell suggested he won't back down on the issue. Treasury Secretary Steve Mnuchin was also on the call and said he supported Toomey.
Democratic lawmakers have criticized Toomey's proposal as unnecessary to the relief bill, arguing it will weaken the Treasury Department and the Fed's ability to fight economic crises.
Sen. Angus King, I-Maine, said on Saturday that Toomey's provision has "nothing to do" with the pandemic and would damage the Biden administration's ability to handle a recession.
— Emma Newburger
FDA chief says extra doses of Moderna vaccine, like Pfizer, could be used if found
Providers will be allowed to administer extra doses of Moderna's coronavirus vaccine if they're found in the vials once the drug is distributed, the chief of the U.S. Food and Drug Administration said in a tweet.
On Wednesday, the FDA acknowledged in a tweet that some vials of Pfizer-BioNTech's Covid-19 vaccine contained an extra full dose — in some cases two extra doses — after the original five that typically come in the vials were used. For the time being, the agency said the extra Pfizer doses could be administered.
Extra shots of Moderna's vaccine will also be fair game if there are any full doses left after the original 10 shots are used, FDA Commissioner Stephen Hahn said in a tweet Saturday.
"Every dose of #COVID19 vaccine makes a difference to overcoming this pandemic," Hahn's tweet read.
During the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices meeting on Saturday, a Moderna representative said that while the company doesn't expect providers to find an 11th dose of its vaccine, a "very skilled" person may be able to occasionally withdraw an extra dose.
— Noah Higgins-Dunn
Bernie Sanders and Chuck Schumer receive vaccine

Senator Bernie Sanders, I-VT, received the Covid-19 vaccine on Saturday, according to a release from his Senate office.
"As the vaccine is being distributed, we must all continue wearing masks and engage in social distancing," Sanders said in a statement. "That is how we will beat this virus and end this terrible pandemic."
Sanders is among several lawmakers in Washington who have received doses of the vaccine and are trying to reassure Americans of its safety.
House Speaker Nancy Pelosi, D-Calif, and Sen. Majority Leader Mitch McConnell, R-Ky., tweeted photos of themselves getting the vaccine on Friday. Sen. Minority Leader Chuck Schumer, D-N.Y., received an injection on Saturday.
"The vaccine is safe and effective, and I encourage everyone to take it as it becomes available," Schumer wrote in a tweet.
Vice President Mike Pence received the vaccine on live television Friday, calling it a "medical miracle" and reassuring the public that they can be confident in its safety and effectiveness. President-elect Joe Biden and his wife, Jill, are set to receive the vaccine on camera on Monday.
— Emma Newburger
CDC advisory panel unanimously backs Moderna vaccine
A panel of experts at the Centers for Disease Control and Prevention has endorsed Moderna's vaccine for people 18 years of age and older in the U.S., clearing another hurdle before shots start on Monday.
The Advisory Committee on Immunization Practices voted 11-0 to recommend the vaccine. Three people on the panel recused themselves.
CDC Director Robert Redfield now has to sign off on the recommendation.
The recommendation comes after the Food and Drug Administration granted emergency use authorization for Moderna's vaccine on Friday.
—Spencer Kimball
Lawmakers expect stimulus deal soon, but final vote may not come until Monday
With the clock ticking toward a government shutdown, some congressional lawmakers are optimistic about a Covid relief deal coming together soon.
Senate Republican Whip John Thune, R-S.D., said on Saturday that a final vote on the relief plan could happen on Sunday but will more likely occur on Monday.
"I'm still somewhat hopeful we could wrap this up if the House moves quickly," Thune said.
A final outstanding issue involves the battle over the Federal Reserve's lending powers. Senator Patrick J. Toomey, R-Pa, is drafting language to block the incoming Biden administration from reviving emergency lending programs, a move that Democrats have condemned.
House Speaker Nancy Pelosi said on Saturday that once arguments over the Fed's lending programs are resolved, the deal could come together quickly and "everything will fall into place."
Treasury Secretary Steven Mnuchin was briefing Republican Senators on Saturday at 1 p.m. ET to provide status updates on negotiations and potentially address the Fed lending issues.
— Emma Newburger
U.K. to impose restrictions ahead of Christmas following new virus strain, prime minister says
British Prime Minister Boris Johnson announced fresh restrictions ahead of the Christmas holiday, saying gatherings can't go ahead and non-essential shops must close in London and much of southern England.
Johnson said that the capital and other areas in southern England currently under Tier 3, the highest level of coronavirus restrictions, will move to an even stricter new Tier 4 that requires non-essential shops, hairdressers and indoor leisure venues to close after the end of business hours Saturday.
A planned five-day easing of socializing restrictions that would allow up to three households to meet in "Christmas bubbles" will be canceled for Tier 4 areas. The new restrictions come after the U.K. identified a new coronavirus strain that "can spread more quickly" than prior strains of the virus, according to England's top medical officer.
"These further restrictions are a bitter blow for Londoners who were hoping to spend time with loved ones safely this Christmas, and for businesses who have already suffered so much this year," London Mayor Sadiq Khan said in a statement following the announcement. Khan called on the government to increase mass testing and provide financial support to businesses and people who are self-employed.
— Noah Higgins-Dunn, The Associated Press
U.S. has allocated 7.9 million vaccine doses for distribution, new shipments arrive Monday
The U.S. has allocated a total 7.9 million doses of Pfizer and Moderna vaccines for distribution across the U.S. with a new round of shipments arriving Monday and continuing through the week, according to U.S. Army General Gustave Perna, who oversees logistics for Operation Warp Speed.
On Friday, Moderna's vaccine received emergency use authorization from the Food and Drug Administration, and the one from BioNTech and Pfizer got it last week.
Perna said the country is on track to allocate 20 million doses by the end of the year.
The doses will go out to more than 3,700 locations across 64 jurisdictions that have ordered vaccines and five federal entities, Perna said. The Moderna and BioNTech-Pfizer vaccines both require two doses per person that must be weeks apart.
Moderna has handed off its vaccine from its manufacturing sites to McKesson, Perna said.
"At McKesson distribution centers, boxes are being packed and loaded today," he said. "Trucks will begin rolling out tomorrow from FedEx and UPS, delivering vaccines and kits to the American people."
The group has already started shipping kits containing needles and syringes for disseminating the vaccine. Going forward, Perna said, those kits will be paired with the vaccines for delivery.
—Jordan Novet
McConnell urges Congress to pass relief, warns delays cost jobs

Senate Majority Leader Mitch McConnell, R-Ky, urged Congress to pass a coronavirus stimulus compromise and said that each day lawmakers delay an agreement costs American jobs and lives.
"There's a gravitational pull here in Congress, where, unless we're careful, any major negotiation can easily slide into an unending catalogue of disagreements," McConnell said on the Senate floor. "Let's guard against that."
Congressional lawmakers are still hammering out final policy disagreements. A short-term spending bill is funding the government through Sunday as leaders maintain they are close to making a deal. However, Congress may not pass a relief plan until next week.
"The American people cannot feed their families or pay their bills with Congress' good faith discussions. They need us to act," McConnell said. "We need to conclude our talks, draft legislation and land this plane."
— Emma Newburger
U.S. Army general apologizes for cuts to vaccine allotments for states
U.S. Army Gen. Gustave Perna, who oversees logistics for President Donald Trump's vaccine program Operation Warp Speed, has apologized for the confusion this week after some state officials reported their allocations of Pfizer vaccines were cut, and it wasn't made clear who was at fault.
"I want to take personal responsibility for the miscommunication," Perna told reporters during a press conference. "I am responsible, and I take responsibility for the miscommunication."
Perna said states asked for planning numbers, but the estimates he gave were higher than the actual number of doses available for release. As a result, he had to lower the allocation numbers.
"It was a planning error, and I am responsible," Perna said. "We're learning from it, we're trying to get better, because at the end of the day it's about facilitating the most available vaccine doses that are releasable out to the American people."
Reporters asked Perna to provide additional details on what caused the error, but the general said he "doesn't know how to explain it any clearer."
The federal government is still on track to allocate roughly 20 million doses to every jurisdiction by the end of the year, Perna said. Distribution of those doses will push into the first week of January, he said.
— Noah Higgins-Dunn
Senate vote on stimulus still days away, Sen. Coons says

Sen. Chris Coons, D-DE, said he believes the Senate will vote to pass the $900 billion Covid-19 recovery package on Monday or Tuesday.
"It is still days away," Coons said on MSNBC. "We frankly should be closing this deal and delivering billions of dollars of assistance to Americans who are facing eviction, hunger, joblessness and homelessness."
Congress on Friday passed a two-day spending bill to avoid a government shutdown after hitting a snag in negotiations. Lawmakers are tying Covid relief and government funding together.
Lawmakers in both chambers have emphasized that they're close to making a deal in the next few days, but Congress faces another deadline of 12:01 a.m. ET Monday to avoid a government shutdown.
— Emma Newburger
U.K. identifies new coronavirus strain that can spread more quickly, alerts the WHO
England's top medical officer announced that the U.K. has identified a new variant of the coronavirus that "can spread more quickly" than prior strains of the virus.
The nation has alerted the World Health Organization and will continue to analyze data on the new strain, said Professor Chris Whitty, England's chief medical officer. However, there's no evidence yet that the contagious new variant causes a higher death rate or affects the vaccines and treatments, though "work is underway to confirm this," Whitty said in a statement.
"Given this latest development it is now more vital than ever that the public continue to take action in their area to reduce transmission," Whitty said.
— Noah Higgins-Dunn
How Moderna's vaccine compares with Pfizer's
Moderna's coronavirus vaccine has now joined Pfizer's drug as the only two Covid-19 vaccines in the U.S. granted emergency authorization from the U.S. Food and Drug Administration.
Both vaccines use messenger RNA, or so called mRNA, technology that teaches the cells to make the virus' spike protein to trigger an immune response. Both vaccines have proven to be highly efficacious; Moderna's drug was 94% effective in preventing Covid while Pfizer's was 95% effective.
Both vaccines will also call for a second trip to the clinic since they each require two doses. However, Moderna's second dose should be given 28 days after the first, while Pfizer's takes 21 days. How old you are will matter: The FDA authorized Pfizer's vaccine for people 16 years old and older, while Moderna's drug was cleared for people 18 and older.
The vaccines come with differing logistical challenges as well. While Pfizer's drug requires a storage temperature of minus 94 degrees Fahrenheit, Moderna's doses can be stored for up to six months at minus 4 degrees Fahrenheit.
— Noah Higgins-Dunn
Where the stimulus talks stand in Congress
Unable to reach a combined Covid relief deal and government funding plan during the week, Congress passed and President Donald Trump signed a two-day funding bill on Friday to avert an immediate government shutdown and give lawmakers more time to iron out their differences.
The House will not meet again and vote until 1 p.m. Sunday at the earliest. Lawmakers have until 12:01 a.m. ET Monday morning to reach a deal, otherwise the government will shut down.
Twelve million Americans are currently set to lose expanded jobless benefits the day after Christmas if a relief deal is not passed. Billions of dollars are also needed to distribute vaccines and shore up the nation's health-care system.
The developing $900 billion Covid relief plan would include the following:
- $300 in supplemental federal unemployment benefits
- At least $300 billion of assistance for small businesses
- $600 stimulus checks for most adults and $600 per child for at least two kids
The deal is also expected to include funding for vaccines, testing, schools, hospitals and transportation, though specific dollar figures haven't been released. It's also unclear what will happen to the federal eviction moratorium, rental assistance, and the student loan payment moratorium.
The $900 billion deal is not expected to include assistance for state and local governments or liability protections for businesses. Those issues have been major sources of contention between Republicans and Democrats, preventing a deal for months.
— Spencer Kimball






